Michelle Howard, PhD, has always been interested in pediatric cancers, but she credits her patients, not her profession, for pushing her and her research team toward the next innovative solution. Howard is leading efforts to develop new treatments for diffuse intrinsic pontine glioma (DIPG), a rare pediatric brain cancer with a median five-year survival rate of only 2%, according to the DIPG/DMG Resource Network.
“Dealing with cancer is often one of the most difficult times in a patient's life. Being able to provide hope through my research is what drives me daily,” says Howard, an assistant professor of radiation oncology at University of Iowa Health Care, member of Holden Comprehensive Cancer Center, and an Institute for Clinical and Translational Science (ICTS) K12 grant awardee. “The pediatric patients I have had the privilege of helping treat have had a profound impact on me, both in my training and in my current clinical position, as these patients are incredibly resilient in the midst of a scary time.”
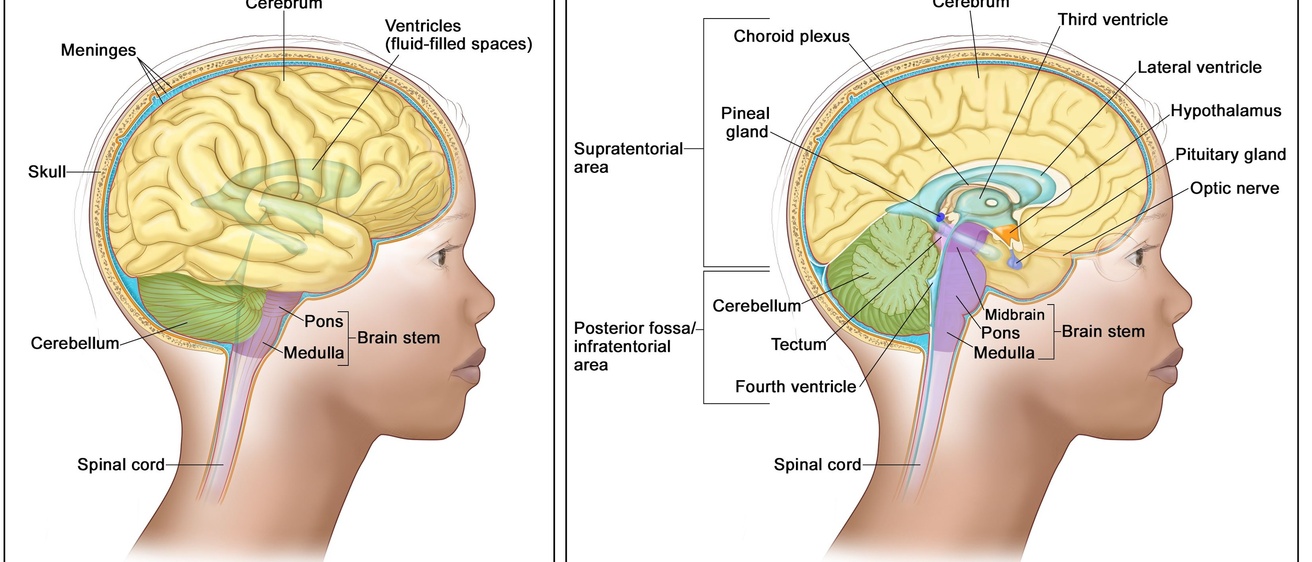
Understanding DIPG
DIPG originates in the bottom portion of the brainstem, specifically in the pons, which controls vital functions such as breathing, heart rate, and blood pressure, and coordinates the nerves necessary for everyday tasks. It is an aggressive cancer, and the disease course for children with DIPG is rapid. Symptoms like facial weakness/paralysis, weakness in arms and legs, headaches, and slurred speech typically start less than four weeks before patients seek medical attention and are diagnosed.
Due to the location of DIPG, surgical removal is often not recommended. Typically, radiation is used to shrink the tumor and temporarily relieve symptoms, but DIPG has no cure. This reality underscores the need for approaches that can meaningfully extend survival for children with DIPG.
Targeting DIPG’s redox vulnerability
In 2023, Howard and her research team found that DIPG cells are sensitive to hydrogen peroxide (H₂O₂) but are also very efficient at removing H₂O₂, which is generated by radiation therapy. With that understanding tied to the existing knowledge on the effectiveness of radiotherapy in shrinking DIPG, the Howard lab developed a new approach to DIPG treatment. In this approach, the team combined radiation with medications that selectively disrupt the DIPG cells’ H₂O₂ removal systems. By using medications that block the cells’ ability to remove the H₂O₂, Howard found they could improve the effectiveness of radiation therapy.
The medications being investigated are Auranofin, an FDA-approved drug originally used to treat rheumatoid arthritis by inhibiting hydrogen peroxide metabolism, and Cu-ATSM, a copper-containing compound that generates excess hydrogen peroxide and is currently being tested in clinical trials for amyotrophic lateral sclerosis (ALS). Together, these drugs overwhelm the cancer cells’ ability to neutralize hydrogen peroxide’s damaging effects and dramatically enhance the sensitivity of DIPG cells to radiation compared to radiation or either drug alone. This combination showed great promise in vivo, slowing DIPG tumor growth compared to the control group.
A scientist on a mission
Howard is pioneering the only research study of DIPG at the UI, and her work is supported by the ICTS K12 grant, a program designed to identify and train outstanding junior faculty on campus who seek a career in clinical research. The goal is for these scholars to develop a successful clinical and translational research career and become independently competitive for other awards.
“This grant protects 75% of my time for research, so I can really focus on building my research group, applying for funding, and progressing the research goals of the lab,” Howard says. “Additionally, through the ICTS educational programming, I have learned about community partnerships and how they can enhance the current work my lab is doing, even as a basic or translational scientist.”
Through the ICTS pilot program, K12 scholars like Howard are paired with community advisory board members (CAB) to create a joint project together to enhance community involvement within their research.
Howard was paired with Kara Boeldt, advocate and the founder of EndPreeclampsia.org, a non-profit maternal health organization. Howard and Boeldt, and others that will participate in the program are paired based on similarities in research interests, community, and/or engagement methods. In this case, Boeldt's efforts managing a community for people experiencing pregnancy complications paired well with Howard’s goal of reaching the community of caregivers of children with DIPG. The pair is working on an additional study with the CAB to gain an understanding of the experiences of DIPG caregivers.
"Information from this study will be used to guide laboratory research focused on improving treatments for DIPG, with the goal of developing safer and more effective therapies that reflect the priorities and needs of patients and families,” Howard adds.
Advancing toward clinical trials
Building on the success of the Auranofin and Cu-ATSM in vivo studies, the next step for Howard involves determining the mechanism of action, completing efficacy studies in a brain-based model, and moving from the lab into human clinical trials at UI. As the impact of Howard’s research grows, she will continue her commitment to patients and move closer to her goal of developing potentially lifesaving new treatment options for DIPG and advancing progress in pediatric cancer research.