Main navigation
Philosophy
The Institute for Clinical and Translational Science Evaluation Core supports clinical and translational science regionally and nationally through programmatic evaluation and routine data capture that support strategic management.
The ICTS Evaluation philosophy is rooted in well established evaluation models such as the Centers for Disease Control Evaluation Model along with contemporary Participatory, Developmental, and Utilization-Focused program evaluation paradigms. We marry these paradigms with the popular Plan, Do, Study, Act (PDSA) model to create an integrated framework for evaluation and Continuous Quality Improvement (CQI).
Plan, Do, Study, Act Model for CQI
Learn more about the PDSA Framework for Program Evaluation and Continuous Quality Improvement.
Evaluation efforts are supported by the program evaluation, and other key ICTS personnel, including module directors, project managers, and the External Advisory Committee.
Impact and Contribution
Our evaluation initiatives are moving from short-term internal performance improvements to data-driven sustained, organization-wide improvements to speed delivery of discovery into practice. ICTS provides services and support to catalyze innovative clinical and translational science that leads to measurable improvements in the health of Iowans and the nation. Selected key accomplishments are highlighted below:
Impact Stories
The Enrollment and Characteristics of Clincal Trials (ECCT) Dashboard
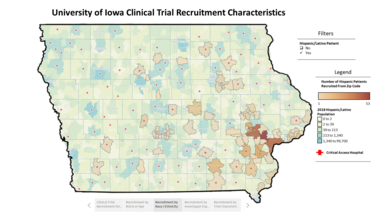
Tracking enrollments in CRU interventional clinical trials has allowed us the opportunity to visualize state demographics overlayed with our successful enrollments into trials. This ECCT dashboard incorporates race/ethnicity/rural-urban codes, age and can be customized for specific data trends.
The ICTS Pilot Grant Program for Clinical and Translational Studies (PILOTS)
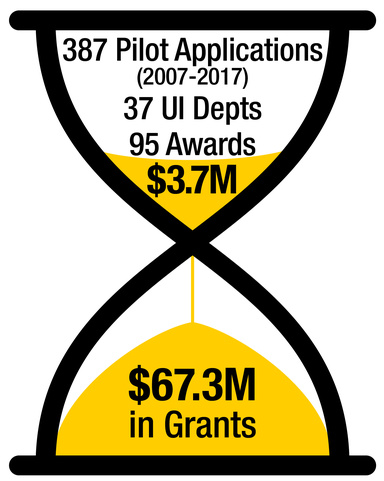
Since 2007 ICTS pilot awardees have received $141 million dollars in external funding demonstrating that recipients of our pilot awards have gone on to direct highly successful CTR programs. Moreover, our PILOTS program awardees have been tremendously successful leading to >3100 publications and >76,000 citations from 2008-2020.
Publications
Publications
Subramain M, O’Quinn DA, Davis H. 4124 An innovative Tool for Completing the Clinical and Translational Science Award (CTSA) Research Performance Progress Report (RPPR) using REDCap. Journal of Clinical and Translational Science. 2020;4(s1):69-70. doi:10.1017/cts.2020.229
Subramain M, Wangui-Verry JM, Sprenger KJ, Comellas AP, Barlow PB. Impact of COVID-19 on Clinical Research Units (CRUs). J Clin Transl Sci. 2021;5(1):e167. Epub 20210813. doi: 10.1017/cts.2021.836. PubMed PMID: 34659802; PMCID: PMC8503072.
Subramain M, Wangui-Verry JM, Sprenger KJ, Ball C, Goins JL, Barlow PB, Comellas AP. 519 The Research Unit Network (RUN) as a Learning Research System. Journal of Clinical and Translational Science. 2022;6(s1):107. doi: http://doi.org/10.1017/cts.2022.313.
Smith TL, Barlow PB, Peters JM, Skolits GJ. Demystifying reflective practice: Using the DATA model to enhance evaluators' professional activities. Eval Program Plann. 2015;52:142-7. Epub 2015/05/22. doi: 10.1016/j.evalprogplan.2015.04.004. PubMed PMID: 26051793.
Barlow, P. B., et al. (2016). Using a Pilot Grant Program and Teamwork Training to Improve the Knowledge and Skills of Science Teams. SciTS. Phoenix, AZ.
Subramain M, Wangui-Verry JM, Sprenger KJ, Comellas AP, Barlow PB. Impact of COVID-19 on Clinical Research Units (CRUs). J Clin Transl Sci. 2021 Aug 13;5(1):e167. doi: 10.1017/cts.2021.836. PMID: 34659802; PMCID: PMC8503072.
Comellas AP, Wangui-Verry JM, Sprenger KJ, Winokur PL, Barlow PB, Subramain M. Research unit network (RUN) as a learning research system. J Clin Transl Sci. 2023 Mar 27;7(1):e89. doi: 10.1017/cts.2023.514. PMID: 37125056; PMCID: PMC10130846
Garg A, Subramain M, Barlow PB, Garvin L, Hoth KF, Dukes K, Hoffman RM, Comellas AP. Patient Experiences with a Tertiary Care Post-COVID-19 Clinic. J Patient Exp. 2023 Jan 17;10:23743735231151539. doi: 10.1177/23743735231151539. PMID: 36698619; PMCID: PMC9869203.
Current Projects
Projects
Common Metrics Initiative
Refining Publication Data Mining Tool to Improve CTSA Hub Impact Tracking
Workforce Development and Informatics Guidelines Authorship
CTSA Team Science Working Group
Analysis of Team Science in Promotion and Tenure Policies Across the CTSA Consortium
SPARC I-CART Clinical Trials Tracking Improvements
Evaluation Research
Promoting Team Science Across a CTSA Hub Institution
Content Analysis of Team Science Pilot Grant Program Applications
Examining the Duration Between IRB Approval and First Patient Accrual
Program Evaluation
STAR Registry Evaluation
Using Dashboard to Drive Hub Decision-Making
Contact Information

Maran Subramain, PhD
- Email: maran-subramain@uiowa.edu
- Phone: (319) 384-8319