The common metrics initiative uses a set of shared measures to help focus all activities across the 62 medical research institutions participating in the Clinical and Translational Science Award (CTSA) Consortium.
The common metrics were collaboratively developed with the National Center for Advancing Translational Sciences (NCATS) and stakeholders from the CTSA program.
Current Metrics
Median IRB Review Duration
The median number of calendar days from the official IRB application date to the official IRB final approval date for fully reviewed protocols submitted to the Iowa IRB.
Pilot Funding Publication and Subsequent Funding
The number and percent of research projects that expended ICTS pilot funding that resulted in one or more publications or in additional funding.
Careers in Clinical and Translational Research
The number and percent of Iowa scholars and trainees who completed the KL2 and TL1 programs, respectively, who are currently engaged in clinical and translational research.
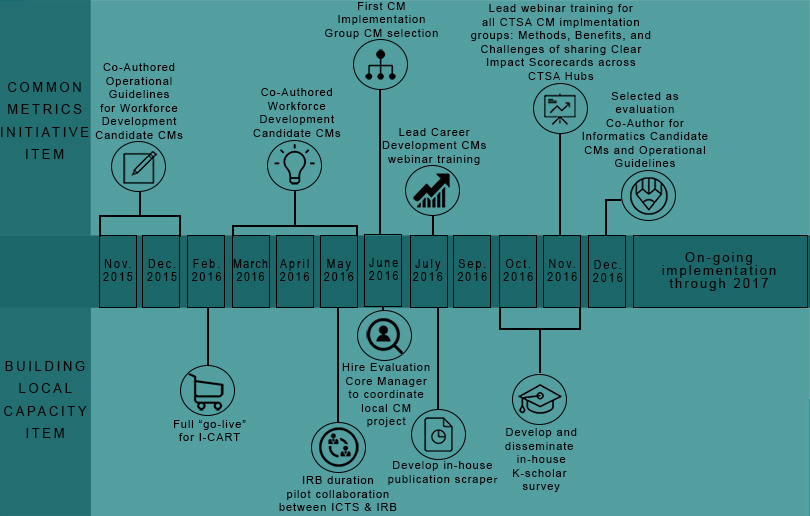
Iowa’s Contribution to the Common Metrics Initiative
ICTS’ Evaluation Core has acted as a collaborative partner since the inception of CTSA Common Metrics by actively participating in the election, refinement, and operationalization of selected metrics.
Help the Institute for Clinical and Translational Science at the University of Iowa continue making new strides in medical research by citing the NIH CTSA program grant UM1TR004403.