Research unit network (RUN) as a learning research system
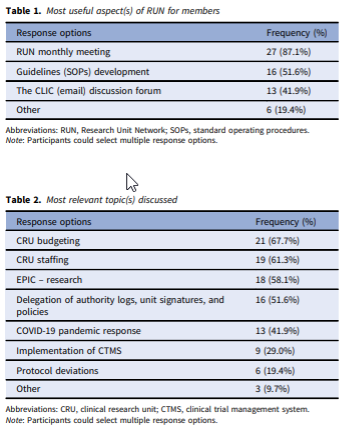
Abstract: The clinical research units (CRUs) are one of the main spaces where both translational research and science take place. However, there is a lack of information about both best practices for CRU operations and, ultimately, benchmarks to evaluate CRU performance. The Research Unit Network (RUN) was created with the purpose to enable direct communication and collaboration among CRUs. An online survey was administered to further illustrate the functionality and impact of RUN. Thirty-one individual survey responses (39.2%) were included in the final analysis. The members value RUN monthly meetings (87.1%) as the most useful aspect of this network and CRU budgeting (67.7%) and staffing (61.3%) were the most relevant topics discussed. This is followed by EPIC – Research (58.1%), delegation of authority logs, unit signatures, and policies (51.6%), COVID-19 pandemic response (41.9%), the implementation of clinical trial management system (29.0%), and protocol deviations (19.4%). The intermediate goal of RUN is to identify best practices CRUs are establishing, implementing, and sharing these experiences with the goal to adopt them in different CRUs. The network’s long-term goal is to establish standard benchmarks that can be used for evaluating the performance of CRUs across the nation.
Please click here for full article: Research unit network (RUN) as a learning research system (cambridge.org)
Impact of COVID-19 on Clinical Research Units (CRUs)
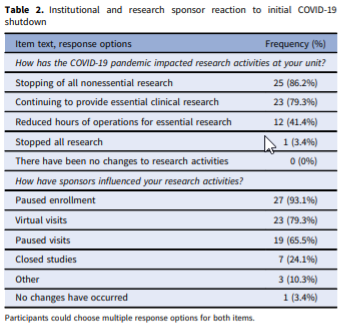
Abstract: Few studies have explored the challenges that the COVID-19 pandemic has presented for Clinical Research Units (CRUs), the solutions that have been implemented, and the changes that have been made in the operational guidelines for these entities. This study sought to identify and document common practices implemented by CRUs around the United States of America (USA) when addressing the unique challenges posed by the COVID-19 pandemic. This descriptive study utilized a non-experimental mixed-methods approach and gathered data from representatives of 43 CRUs across the USA. An online survey was followed by in-depth interviews. The findings show that challenges faced from the COVID-19 pandemic, changes made to daily operations, and lessons learned are very similar across CRUs. Although most CRUs never stopped performing essential clinical research, many adapted to the pandemic by engaging in virtual visits, and many played key roles in administering and supporting both COVID-19 therapeutic and vaccine trials. Follow-up interviews showed that processes for formal approval and reopening were similar across CRUs. In addition to highlighting the significance of the role played by CRUs during the COVID-19 pandemic, this study addresses the relevance of CRUs and lays the groundwork for future conversations on the importance of these units.
Please click here for full article: Impact of COVID-19 on Clinical Research Units (CRUs) (cambridge.org)
Patient Experiences with a Tertiary Care Post-COVID-19 Clinic
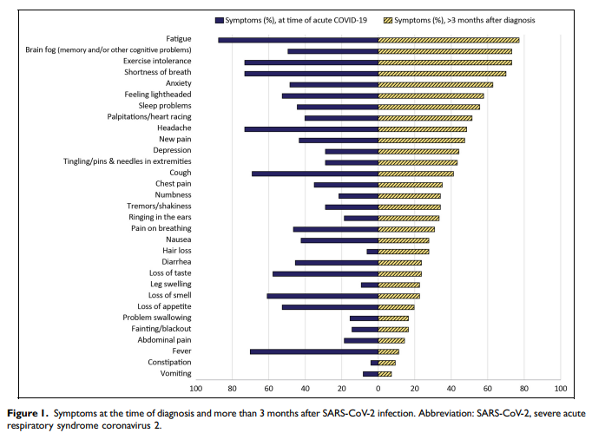
Abstract: Post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PASC) is a complex condition with multisystem involvement. We assessed patients’ experience with a PASC clinic established at University of Iowa in June 2020. A survey was electronically mailed in June 2021 asking about (1) symptoms and their impact on functional domains using the Patient-Reported Outcomes Measurement Information System (PROMIS) measures (Global Health and Cognitive Function Abilities) (2) satisfaction with clinic services, referrals, barriers to care, and recommended support resources. Survey completion rate was 35% (97/277). Majority were women (67%), Caucasian (93%), and were not hospitalized (76%) during acute COVID-19. As many as 50% reported wait time between 1 and 3 months, 40% traveled >1 h for an appointment and referred to various subspecialities. Participants reported high symptom burden-fatigue (77%), “brain fog” (73%), exercise intolerance (73%), anxiety (63%), sleep difficulties (56%) and depression (44%). On PROMIS measures, some patients scored significantly low (≥1.5 SD below mean) in physical (22.7%), mental (15.9%), and cognitive (17.6%) domains. Approximately 61% to 93% of participants were satisfied with clinical services. Qualitative analysis added insight to their experience with healthcare. Participants suggested potential strategies for optimizing recovery, including continuity of care, a co-located multispecialty clinic, and receiving timely information from emerging research. Participants appreciated that physicians validated their symptoms and provided continuity of care and access to specialists.
Please click here for full article: Patient Experiences with a Tertiary Care Post-COVID-19 Clinic - PMC
Understanding enterprise data warehouses to support clinical and translational research: enterprise information technology relationships, data governance, workforce, and cloud computing.
Objective: Among National Institutes of Health Clinical and Translational Science Award (CTSA) hubs, effective approaches for enterprise data warehouses for research (EDW4R) development, maintenance, and sustainability remain unclear. The goal of this qualitative study was to understand CTSA EDW4R operations within the broader contexts of academic medical centers and technology.
Please click here for full article: https://academic.oup.com/jamia/article/29/4/671/6433095
Dissemination and continuous improvement of a CTSA-based software platform, SPARCRequest©, using an open source governance model
Abstract: SPARCRequest© (Services, Pricing, & Application for Research Centers) is a web-based research management system that provides a modular and adaptable “electronic storefront” for research-related services. Developed by the South Carolina Clinical & Translational Research Institute at the Medical University of South Carolina, it was released as open source (OS) code in 2014. The adoption of SPARCRequest© accelerated in 2016, when, to ensure responsiveness to the needs of partners, its governance also became open. This governance model enables OS partners to suggest and prioritize features for new releases. As a result, the software code has become more modularized and can be easily customized to meet the diverse needs of adopting hubs. This article describes innovative aspects of the OS governance model, including a multi-institutional committee structure to set strategic vision, make operational decisions, and develop technical solutions; a virtual roadmap that ensures transparency and aligns adopters with release-based goals; and a business process model that provides a robust voting mechanism for prioritizing new features while also enabling fast-paced bug fixes. OS software evolves best in open governance environments. OS governance has made SPARCRequest© more responsive to user needs, attracted more adopters, and increased the proportion of code contributed by adopters.
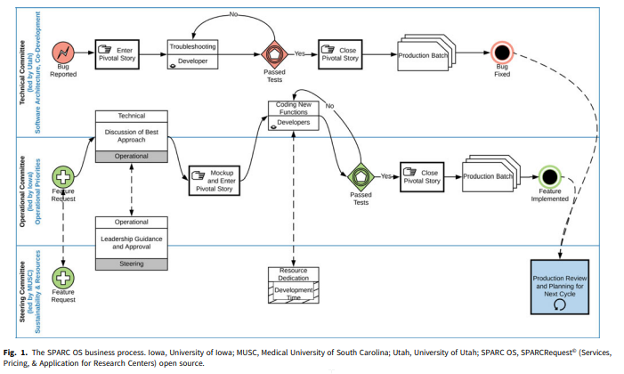
Please click here for full article: Dissemination and continuous improvement of a CTSA-based software platform, SPARCRequest©, using an open source governance model (cambridge.org)
Research IT maturity models for academic health centers: Early development and initial evaluation.
Abstract: This paper proposes the creation and application of maturity models to guide institutional strategic investment in research informatics and information technology (research IT) and to provide the ability to measure readiness for clinical and research infrastructure as well as sustainability of expertise. Conducting effective and efficient research in health science increasingly relies upon robust research IT systems and capabilities. Academic health centers are increasing investments in health IT systems to address operational pressures, including rapidly growing data, technological advances, and increasing security and regulatory challenges associated with data access requirements. Current approaches for planning and investment in research IT infrastructure vary across institutions and lack comparable guidance for evaluating investments, resulting in inconsistent approaches to research IT implementation across peer academic health centers as well as uncertainty in linking research IT investments to institutional goals. Maturity models address these issues through coupling the assessment of current organizational state with readiness for deployment of potential research IT investment, which can inform leadership strategy. Pilot work in maturity model development has ranged from using them as a catalyst for engaging medical school IT leaders in planning at a single institution to developing initial maturity indices that have been applied and refined across peer medical schools.
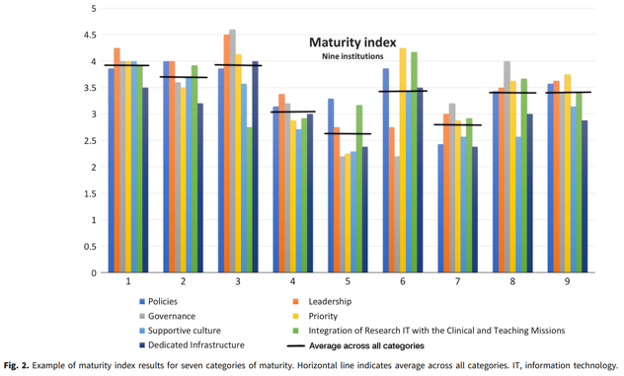
Please click here for full article: CUP_CTS_1800339 1..6 (cambridge.org)